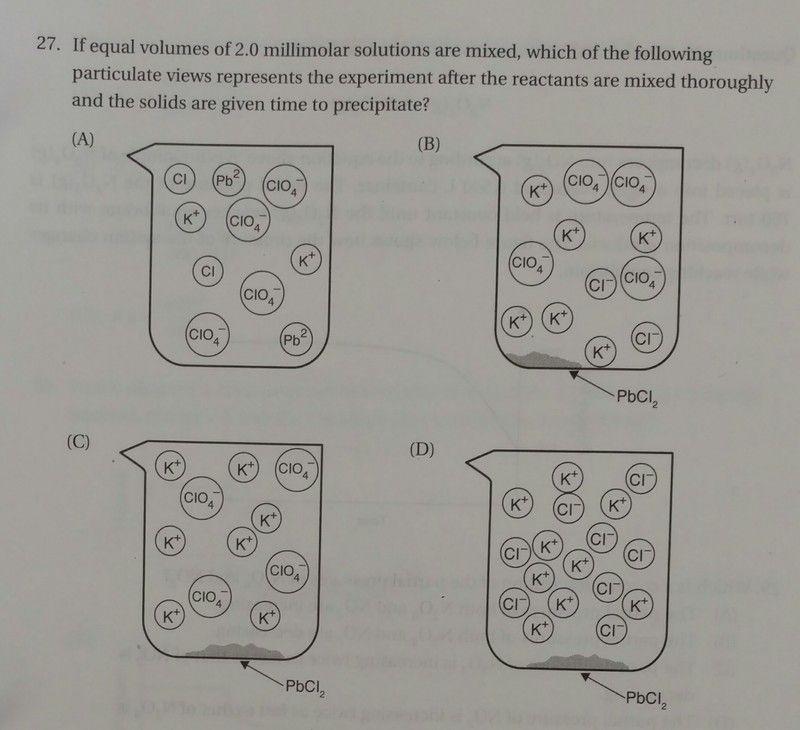
Nothing forms. That’s the whole point of the question. The question is testing whether or not the student recognizes that no reaction occurs (i.e. that no precipitate forms because the concentrations are too low to cause the precipitation).
That’s because the lead ions aren’t doing anything (in the sense that they’re not reacting; obviously they’re continuing to move around randomly due to Brownian motion).
I don’t know why a peroxide would form. Lead (II) chloride, lead (II) perchlorate, potassium chloride, and potassium perchlorate are all stable compounds (certainly more stable than a peroxide would be).
Not that I’m aware of. Perchlorate is stable in aqueous solution.
I doubt it. If the intent were to assess the student’s memory of whether or not lead (II) chloride is insoluble, then either no concentrations would be given or the concentrations would be much higher (closer to 0.1 M). At those concentrations, a precipitate would indeed form. However, the concentrations are probably given for a specific reason: so that students can perform the Qsp calculation. Presumably the Ksp is also included in the test somewhere for the students to compare to their calculated Qsp.
It looks to me that there was simply an error made in sizing the circles such that they don’t cut off the superscripted charges. With the assumption that the Ksp was given in some data table (reasonable considering that this was taken from a practice test) and the assumption that the circles surrounding the Pb[sup]2+[/sup] and Cl[sup]-[/sup] ions are simply formatted too small and therefore cut off a portion of the text, answer choice (A) is best.
Even without making these assumptions, I would still argue that answer choice (A) is best. Let’s assume that the precipitation of lead (II) chloride goes to completion. That is, lead (II) chloride precipitates until either the lead (II) ion concentration or the chloride concentration is zero (or both, if they’re in a stoichiometric ratio). We’ve mixed equal quantities of 0.002 M Pb(ClO[sub]4[/sub])[sub]2[/sub] and 0.002 M KCl. This means that immediately after mixing and just before reaction we have the following concentrations: [Pb[sup]2+[/sup]] = 0.001 M; [K[sup]+[/sup]] = 0.001 M; [ClO[sub]4[/sub][sup]-[/sup]] = 0.002 M; [Cl[sup]-[/sup]] = 0.001 M. After the reaction (as much PbCl[sub]2[/sub] has precipitated as possible), the concentrations are now: [Pb[sup]2+[/sup]] = 0.0005 M; [K[sup]+[/sup]] = 0.001 M; [ClO[sub]4[/sub][sup]-[/sup]] = 0.002 M; [Cl[sup]-[/sup]] = 0 M. That is, all the chloride ions have precipitated and half the lead (II) ions have precipitated. So if our assumption that the precipitation occurs is correct, we need to look for an answer choice where we have X lead (II) ions, 2X potassium ions, and 4X perchlorate ions. There is no such picture shown.
In (D), the perchlorate ions have simply vanished from solution, thereby violating mass balance. In (C), the potassium ion concentration is higher than that of perchlorate. In (B), we have no lead (II) ions left in solution, fewer perchlorate ions than potassium ions, and some chloride ions left in solution. Whereas in (A), we have the exactly correct ratios of ions (albeit with incorrect charges on the lead and chloride ions). Occam’s Razor suggests that it’s simply a formatting error.