Basically I am wondering if atoms go through an evolution of sorts, do they all start out the same? I know they decay but I am more concerned about how they start and form all of the elements.
For the most part, they gets formed in stars. Hydrogen to Helium and so forth on up. They gets spread around when the stars go boom. It’s still mostly hydrogen at that point but lots of other stuff. And new stars (and their solar systems) are formed from that material. The original hydrogen mostly formed in the aftermath of the Big Bang.
Our own sun is a 3rd generation star, i.e. the material forming our solar system came from the result of 2 previous generations of this process. Otherwise, there wouldn’t be so much iron and carbon and lead and so forth all around.
This is an enormous topic that occupied many of the best minds of the 19th and 20th Century, that is now pretty well understood.
The Big Bang created the three lightest (lowest number of protons) elements - hydrogen, helium, and lithium. Hydrogen was the most common, followed by helium, then lithium. We infer this from observations of places that we think have not changed much since the big bang, such as certain dwarf galaxies.
The heavier elements have been built up in a few ways. First, in the interior of stars, elements such as hydrogen and helium are fused to create elements such as carbon, oxygen, and nitrogen among others. Second heavier elements such as iron are created in the explosions of very large stars. Finally, the very heaviest elements are created in the explosive merger of neutron stars, which is something we have learned fairly recently, I believe.
Heavy elements tend to be unstble and can spontaneously divide into ligher duaghter elements.
According to the current theory, the Big Bang formed the nuclei of a large amount of a very few light elements:
Stars condensed out of those primeval elements (mostly hydrogen) and created most of the rest of the elements by fusion reactions:
This process works for elements of increasing massiveness up to iron. For elements more massive than iron, catastropic end-of-star-life events are needed to get past the “iron peak” and create the more massive elements.
TL;DR: most atoms we encounter were created by the fusion of primeval atoms.
If you took all the components out of an atom and weighed them separately would it have the same mass as the atom?
That’s a separate question and not one easily answered.
But generally, no they wouldn’t mass the same if we use the word ‘mass’ the way we typically do.
There’s energy involved in holding those protons and neutrons together. And at those scales, that means E=mc^2 is very relevant. To separate the components requires some energy, i.e. changing the overall mass (equal to the energy by mc^2) of the system. The difference in mass/energy varies by atom and how many protons and neutrons are involved.
There’s still overall conservation of mass/energy but we need to take care to understand ‘mass’ and ‘energy’ are the same kind of thing at atomic scales.
ETA: note this process is what drives life on earth. Nuclear fusion releases energy - as it happens by the same amount as difference in mass we’d observe between the resulting helium and the constituent hydrogen atoms that got smashed together.
Also, not all elements decay. Some elements (or more precisely, some isotopes of some elements) are stable, and some isotopes are unstable. Leave an unstable isotope alone for long enough (and just how long is “long enough” varies, and can be anywhere from a tiny fraction of a second to billions of years or more), and it’ll decay to something else. Leave a stable one alone, and it’ll just sit there, no matter how long you wait.
Off hand, wouldn’t you lose some mass due to change in the amount of energy?
Any updates on if proton decay is a thing?
Yes. If you disassembled an atom into electrons and nucleons, you’d lose some mass (<1%). If you (somehow) disassembled the nucleons into quarks, you’d lose almost all the mass (~99%). Basically, almost all the mass in matter is from binding energy, not the mass of the component parts.
It was once thought that virtually all the elements heavier than iron were created in supernovae. AIUI, when they got around to doing the math, astrophysicists had trouble figuring out how some of those heavy elements were created in SNe.
Then a few years ago, a kilonova was observed. A kilonova is a collision between two neutron stars or a neutron star and a black hole. Those were found to spread a lot of bits and pieces around. And it turns out that many of the heavier elements, including gold, for example, came from those bits and pieces.
Almost all the mass in atoms is embodied in the binding energy keeping the quarks together. A tiny bit of that leaks out and gets called the residual strong force, which binds the nucleons together.
A shift in the amount of force needed to hold things together means there is a shift in the energy and hence mass present.
Famously iron requires the least binding energy and sits at bottom of the fusion and fission pathways. Both lighter and heavier elements need proportionally more binding energy to hold together and are thus proportionally heavier for the number of comprising nucleons.
What is fun to consider is that we are comprised almost entirely of this binding energy and are almost entirely empty space. Even inside of he nucleus.
The early nucleosynthesis work was done by Fred Hoyle. IMHO Nobel winning work, but he rather alienated the physics community with his dogged adherence to the steady state theory of the cosmos and public ridicule of the Big Bang (which he named). Science fiction writing and pushing the panspermia theory of life probably didn’t help either. Awarding a Nobel would tend to be viewed as endorsement of all his theories.
That is very odd!! Why would such a bright guy go off like that. Did his ego drive him mad?
The trick is to go nuts after you’ve gotten the Nobel prize, like Linus Pauling or James Watson.
I met him once. He really seemed sane, even charming. At the time, his steady-state universe idea could not really be refuted. It was the discovery of the cosmic background radiation that did it in and, IIRC, he acknowledged that fact. The temperature of roughly 3 degrees had been predicted and matched the observation. The steady state universe just could not make such a prediction.
I doubt that. Rather the converse I suspect. He wrote good hard science fiction. A for Andromeda is very good and worth reading even now. He believed the steady state model. Panspermia is in some ways a matter of scientists getting outside of their area of expertise, but ironically is an idea that has never gone away. But he became a proponent of viruses from outer space, and other silliness. He developed a whole slew of alternative theories.
If he had a huge enough ego to believe he deserved a Nobel he would be more likely to play the game. He likely didn’t care. Well maybe.
Nucleosynthesis won his co-worker Willian Fowler a share of a Nobel, so it isn’t as if the work wasn’t overlooked. But Hoyle was.
But he was abrasive and enjoyed being a maverick.
He is quoted as saying “it is better to be interesting and wrong than boring and right”.
So, IMHO, a distaste for his maverick personality swayed the Nobel committee away from rewarding a true groundbreaking contribution. Such is life. In some ways I can’t blame them. But it remains a shame.
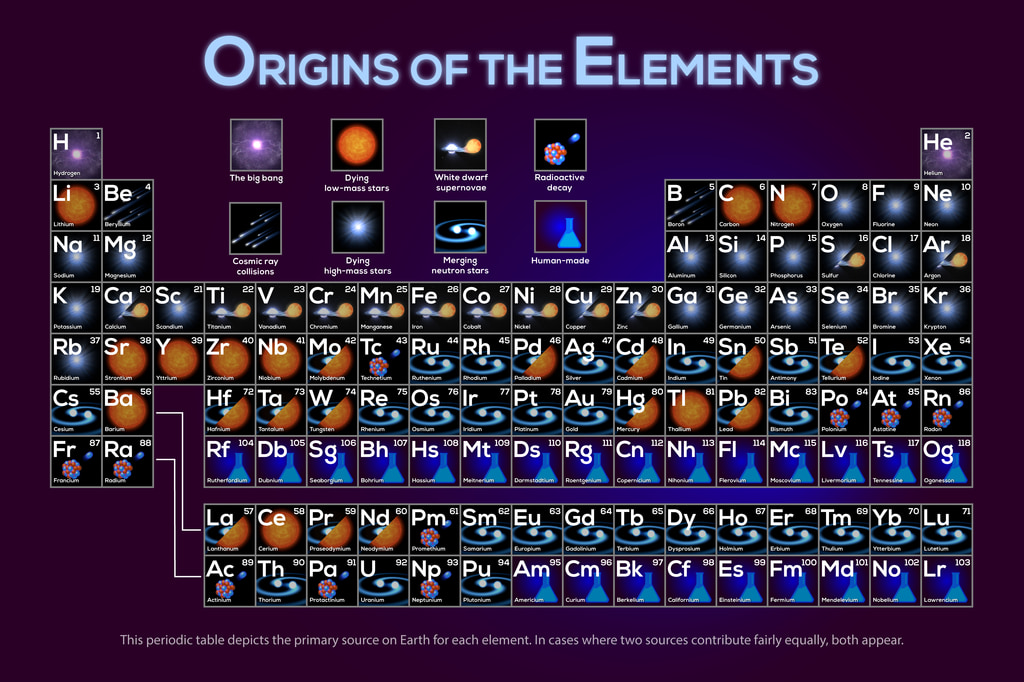
Here’s a periodic table of element origins:
There’ve been several versions of this over time. For instance, the earliest version didn’t have the neutron star mergers. Or the cosmic ray collisions and dying low mass stars. This version also doesn’t show minor contributions. For instance, a small fraction of lithium was made in the Big Bang, but it doesn’t show here.
My favourite of his SF is actually the first: The Black Cloud.
The tech is very outdated now but most of the physics is still good.
It’s true he was one of the originators of Steady State (damn, I always liked that theory myself) but was still enough of a scientist to realize when observational evidence disproved it, I think.
He did go off the rails later in life, but as we have noted, so did a lot of scientists who did outstanding earlier work. Dirac had some very odd ideas in his elder years; William Crookes fell prey to spiritualism etc…
Wikipedia has some info about that.
It looks as if it has been shown experimentally that the lower bound of the lifespan is at least 10^34 years.
Also, the GUT and supersymmetry theories which predicted proton decay seem to be rather out of favour these days…?
One of my favourites too, I like October the First is too Late and The Inferno too.